Car T Cells Upsc
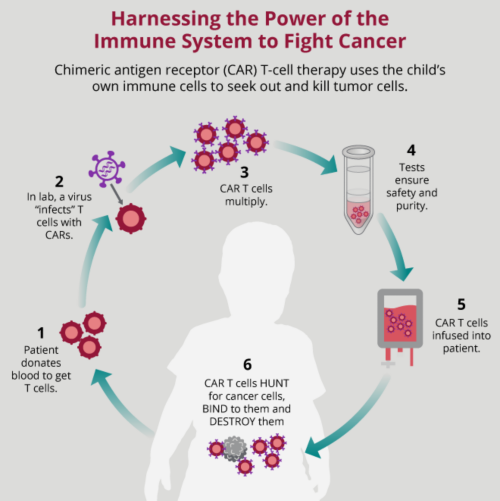
Chimeric antigen receptor car t cell therapy involves genetic modification of patient s autologous t cells to express a car specific for a tumor antigen following by ex vivo cell expansion and re infusion back to the patient.
Car t cells upsc. While these particular histologies represent a rather small number of cases. Kyoto and osaka japan july 16 2019 the center for ips cell research and application cira at kyoto university and takeda pharmaceutical company limited tse 4502 nyse tak takeda today announced that a novel induced pluripotent stem ips cell derived chimeric antigen receptor car t cell therapy icart has been transferred from their t cira research collaboration to takeda as the program begins process development toward clinical testing. Chimeric antigen receptor t cells also known as car t cells are t cells that have been genetically engineered to produce an artificial t cell receptor for use in immunotherapy. As its name implies the backbone of car t cell therapy is t cells which are often called the workhorses of the immune system because of their critical role in orchestrating the immune response and killing cells infected by pathogens.
Chimeric antigen receptors cars also known as chimeric immunoreceptors chimeric t cell receptors or artificial t cell receptors are receptor proteins that have been engineered to give t cells the new ability to target a specific protein. However 10 to 15 of women will present with uterine papillary serous carcinoma upsc or clear cell carcinoma cc. 5b top confirming that chain i by itself does not trigger t cell activation. Upmc hillman cancer center currently offers two types of fda approved car t cell therapy.
On the other hand the anti cd19 control car efficiently lysed nalm6 target cells independently of a1120 as expected. Cars are fusion proteins of a selected single chain fragment variable from a specific monoclonal antibody and one or more t cell receptor intracellular signaling domains. This dual car t cell a new type of car t cell was made by engineering two cars into a single t cell. Each car had a cd4 protein that allowed it to target hiv infected cells and a costimulatory.
The therapy requires drawing blood from patients and separating out the t cells. Under the terms of the t cira. Doctors take a type of white blood cell from your body and genetically. Fda approved car t cell therapies chimeric antigen receptor car t cell therapy is a type of immunotherapy that uses a patient s own genetically modified t cells to find and kill cancer.